RegNav, by Element
Overview
I Joined an R&D project at the early stages of MVP, leveraging digital solutions to navigate medical device regulation and compliance.
I drove the design process from discovery to development, creating robust foundations with a scalable design system.
Core sections include the Establishment Phase, Classification, Static Compliance Plan, Market Fit Pivot, and Product-Level Requirements.
Outcome
We successfully launched RegNav, an AI-powered regulatory intelligence platform that provides MedTech companies with comprehensive Compliance Plans.
Secured initial paying customers and established robust foundations that informed a strategic market fit pivot to the Enterprise segment.
Positioned the company for future lifecycle support and growth.
Role
Sr Product Designer
UX/UI/Research
Discovery
User Testing
Worked with
Directors
Product Managers
Developers
Regulation Experts
Data Scientists
Brand
Element Materials Technology
RegNav
Who Are Element?
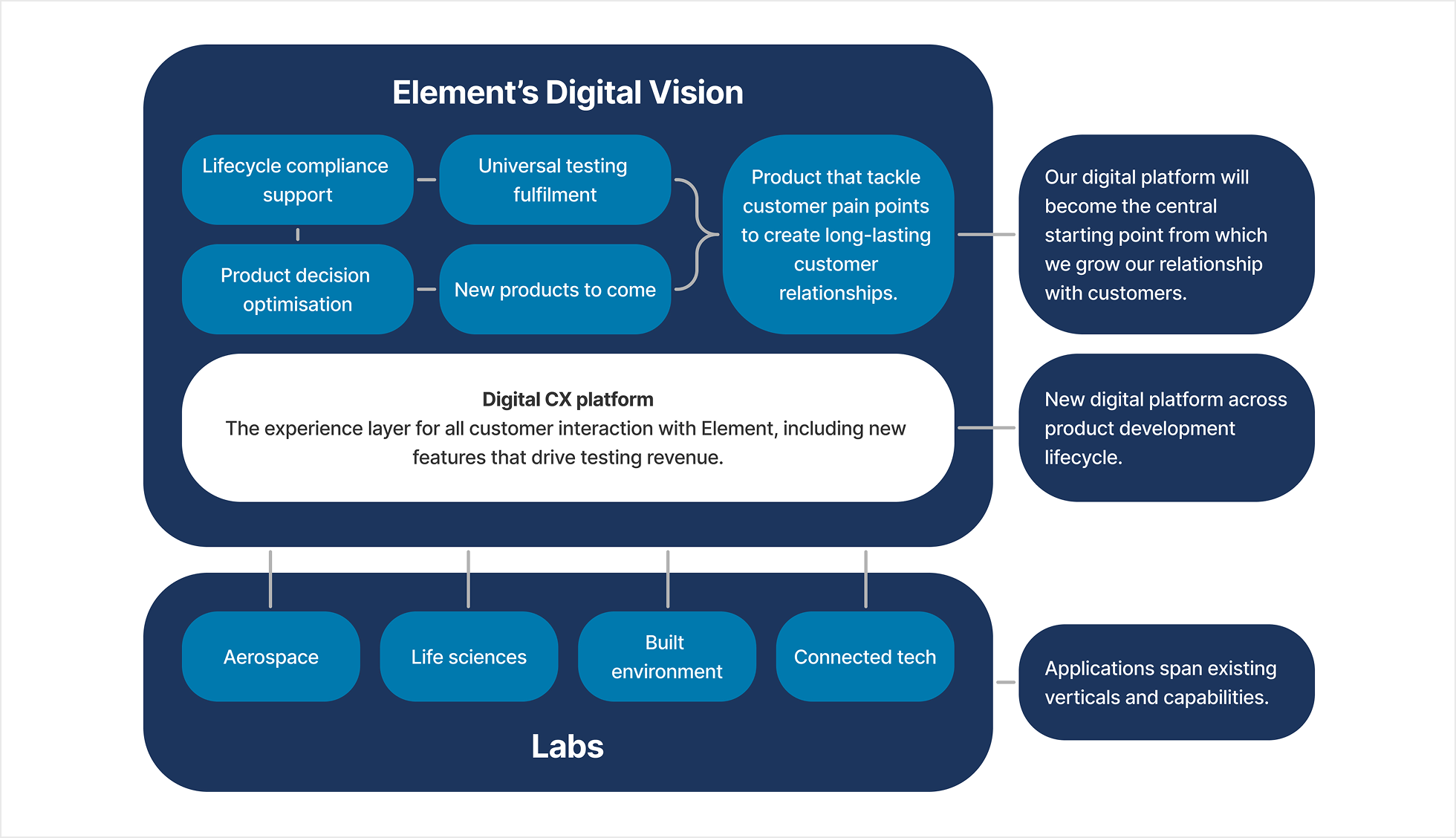
They have nearly 200 years experience in testing with 270 labs in 30 countries, 9K employees, 55K customers, and $1.5BN in non-digital revenue. Element operates in 4 key areas: Life Sciences (pharmaceuticals and medical devices), Connected Tech (mobile phones), Built Environment and Aerospace.
Elements’s Vision
To position the company very differently from a traditional TIC player (Testing, Inspection, Certificate), providing a digital customer experience, that drives testing revenue.
Why RegNav?
Problem Statement
Using Life Sciences as a springboard, MedTech companies face significant challenges, due to the resource-intensive regulatory and testing phase, particularly Class III and De-Novo devices. This can lead to extended time-to-market (2-7 years) and high costs ($2-10M+ per device), resulting in lost revenue and low success rates.
Key challenges:
Navigating complexity: Difficulty in accurately determining necessary examinations, device classifications, and applicable Standards.
Time consuming and costly: Manually reviewing over 1500 Standards and compiling 3000+ pages of documentation can take over 150 hours per device.
Human error: Manual processes are prone to human error and inconsistency, which can lead to significant delays and complications in the regulatory process.
Accessing expertise: Challenges in identifying and securing qualified regulatory consultants, leading to potential delays and inaccuracies.
Maintaining compliance: Ongoing challenges in staying updated on regulatory changes and predicate device updates after product launch, risking non-compliance.
Element VS Competitors
RegNav’s competitive advantage is integrating digital Compliance Plans with Element’s physical testing strategy.
List of players and our competitive offering.
Segments & Value
Startups (0-10 products):
Reduced market entry costs.
Faster time to market.
Accurate Compliance Plans.
SMEs (2-50 products):
Faster time to market.
Scale efficiently
Accurate Compliance Plans.
Enterprises (100+ products):
Reduced product launch risk.
Lifecycle focused.
Compliance Plan accuracy checks.
Breakdown of segments.
The initial focus was on Startups, benefitting from their:
Motivation: Willingness to try new digital solutions, affected by high costs and long time lines.
Simpler needs: Opting for an ‘out-of-the-box’ platform.
Value proposition: Needing assistance in creating a clear Compliance Plan, reducing a significant burden.
Establishment Phase
Agency Context
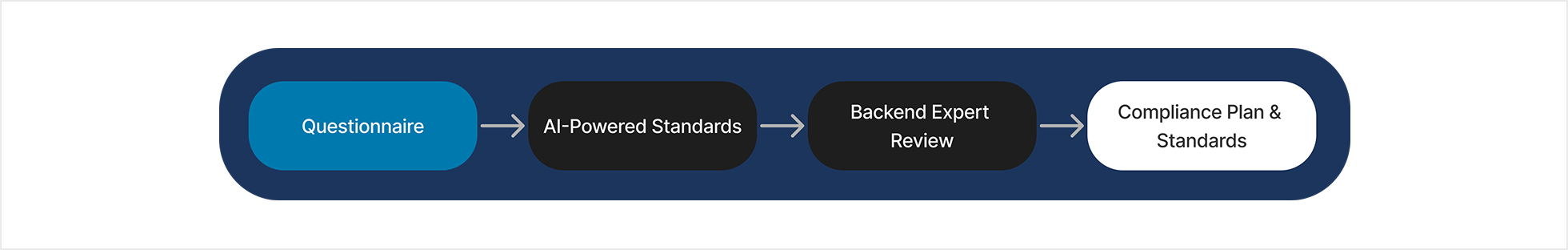
An agency had already begun developing RegPath (later renamed RegNav), an AI-powered platform aimed at helping MedTech Startups accelerate their time-to-market, at a reduced cost. The initial functionality relied on questionnaires, and backend expert review to identify regulatory Standards.
First In-House Designer
Reviewed existing design decisions.
Took ownership of design assets (Figma).
Acquired domain knowledge (MedTech development cycle).
Co-owned the research space (Dovetail/Notion).
Kicked-off a design system with developers (Storybook).
Priority Enhancements
Workflow Redesign: A navigation and workflow improvement project, that addressed user testing insights.
Visual Identity: A small visual identity project, that addressed Global Marketing requirements.
Workflow Redesign
Problem Statement
User testing revealed confusion between the input and output stages of the workflow.
The horizontal tabs layout limited future feature expansion.
My Approach
I visually mapped the existing user flow to pinpoint issues, and experimented with new step groupings for clarity.
I iteratively shared designs and taxonomy with stakeholders to improve the language and layout from the existing UI to the final version.
Vertical Navigation/Stepper
Information hierarchy: Established a new structure to better differentiate the user's input and output stages.
Input - Provide Device Details (About, Classification, Characteristics, Review Essential Principles, Request Compliance Plan).
Output - Compliance Plan (Overview, Standards, Essential Principles)
Language simplification: Introduced clear section titles and removed redundant copy.
Visual guidance: Introduced iconography, progress indicators, and consistent CTAs for improved clarity and guidance.
Legibility & accessibility: Replaced the font (FreightSans with Inter), and optimised the tonal palettes to adhere to WCAG guidelines.
Visual design: Removed unnecessary visual clutter to establish clean grid systems and a more scalable UI.
Future proofing: Created scalable components and logical breadcrumbs.
Visual Identity
Problem Statement
Global marketing required us to establish RegPath as a product within the Element brand.
RegNav Identity
Brand identity: An overarching Element branded bar and a new RegNav logo to establish the product's identity.
UI legibility: Replaced the font (FreightSans Pro with Inter) and optimised the tonal palette to adhere to WCAG guidelines.
Design System
Reviewed open source library (Radix), and removed redundant components.
Created a Figma UI kit, documenting variables (typography, spacing, colour), and sub components.
Connected Figma and Storybook, and implemented the design system.
Established a process for refining, expanding, and developing custom components.
Improved design efficiency, consistency, and established UX/UI principles.
-
Simplification over complexity: Break down a complex process into manageable, user-friendly steps to reduce cognitive load.
Scalability and adaptability: Build a flexible design framework that can grow with the product, and easily accommodate new user insights.
Real world concepts: Use intuitive language, and familiar concepts.
Functional and lean UI: Prioritise a minimalist interface, where every component serves a clear function, and free from irrelevant information.
Visible and explainable: Keep users informed of status, and clearly communicate the why to build trust.
Clarity through visual hierarchy: Use typography, spacing and colour to guide the users eye, and make complex information easier to understand.
Establishment Phase Summary
My initial months focused on a crucial learning exercise, immersing myself in the complex MedTech domain and forming strong foundations as we moved away from the agency model.
Platform mapping: Understood current pain points and the interconnections of the different product sections.
Source of truth: Co-owned the user research space, setting up discovery workshops, testing scripts, and a shared repository of insights (Dovetail AI/Teams).
Lean design approach: Prioritised simple, functional UI components, and core UX patterns.
Scalable design system: Allowed for systematic growth and consistency, enabling us adapt quickly as we learned more about the domain and user needs.
Centralised product copy: Facilitated collaborative feedback, ensuring all language was legally compliant and human-friendly. This process was supported by an AI persona prompt, allowing us to quickly check the copy and maintain a consistent tone.
Classification
Problem Statement
Observational user testing ('Friends & Family' network) revealed users struggling with the Classification step, specifically when assigning their Submission Path, Class, and Product Code. This is an essential step for compliance, determining FDA review pathways, and device comparisons.
Key requirements:
Streamline classification assignment: Enable users to confidently select their Submission Path, Class, and Product Codes by providing clear, informative guidance.
Support multiple codes: Allow users to add multiple Product Codes, designate a primary code, and provide the Experts with more information in the backend.
Classification bypass: Provide an option for users to skip the step if they cannot identify a relevant code.
Ideate
Building on the Navigation/Stepper enhancements, I explored the 'Classification' section, creating flows, prototypes to facilitate team discussions.
Progressive Disclosure Concept
Guided users through hierarchical steps, presenting in-depth contextual information, to inform choices and filtered product code options.
For users who are unable to follow the linear flow, I provided an alternative path featuring an open product code search dialog.
Pivot
Prototyping revealed a key problem, the hierarchical approach created classification conflicts, where answering a question incorrectly filtered out expected results for the user.
The frustrating user experience exposed data repetition, that led to a new opportunity for automation.
We decided to shift away from the hierarchical approach and further explore the open search pathway.
Define & Build
Product Code Finder
A streamlined workflow, prioritising a comprehensive search interface with detailed information, that automates classification assignment by capturing product code data.
Intelligent search: Word match search, with planned semantic search improvement.
Filtering: Comprehensive filters (Class, Submission Type, Medical Specialty, Device Function).
Extensive product code details: Structured, scannable product code information (Definition, Physical State, Technical Method, Regulations).
Information side-panel: In-app details on classification types, with external links.
Product code management: Add multiple codes, compare codes, assign a primary code, and cross reference with their intended use.
Automation: Accurate Submission Path and Class assignment via product code data.
Secondary pathways: Bypass without code identification, expandable content on zero results, and landing screen.
Measure
We conducted observational user testing across all RegNav stages, including Payments, Provide Device Details (About, Classification, Characteristics, Essential Principles Review), and Compliance Plan requests. We documented and analysed the sessions using Dovetail AI, then shared the key takeaways and recommended actions with the wider team on Microsoft Teams.
Dovetail user testing documentation.
Dovetail user testing notes and labels.
Quote Examples
“It's in that area where it's not detailed enough for someone who is really new at this - itʼs too general for that. But someone who has experience will look at this and say of course!ˮ
“If someone was brand new… they might not know that the device could have many classifications or many codes”
Classification Takeaways
User distinction: Recognised a clear difference between experienced Enterprise users who knew their product code and less experienced users who search by device description.
System configuration: Needs to make it more obvious that multiple product codes can be added, and clarify the distinction between a standalone product and a system configuration.
Additional data source: Explore k-documents as another source of data, including information about a device and its substantial equivalence to a predicate device.
Business Impact
The Product Code Finder streamlined the Classification step, and provided more accurate results for the expert review, directly contributing to the reduction of time to create a Compliance Plan from 150 hours to 90 hours.
This work sparked discussions on new lifecycle features, such as the "Predicate Finder," highlighting opportunities for future product expansion.
It was also used as a marketing teaser, revealing low site engagement metrics (Amplitude), that when combined with user insights, helped inform a significant market fit pivot.
Static Compliance Plan
Problem Statement
We lacked a customer-facing Compliance Plan that included the necessary educational material and branding to justify its $10,000 price tag. This was complicated by the fact that dev resources were unavailable, due to optimising the user input workflow. While our existing Excel version functionally served the purpose for managing the data, it couldn't meet the education and branding needs, so leadership proposed a static PDF as a temporary solution.
Recognising the technical limitations of a static document, I strategically positioned the PDF as a valuable research tool. This allowed us to proactively gather early insights on user needs, informing the future development of an online version.
What is a Compliance Plan?
A medical device Compliance Plan is a documented system to ensure product safety, and regulatory adherence throughout its lifecycle.
Overview: Product details (classification, use).
Standards: Device safety/performance evaluation methods.
Essential Principles: Design/manufacturing requirements.
Product Codes: FDA identifiers for tracking.
Predicates: Legally marketed device for comparison.
Static PDF Version
To understand the core components of a Compliance Plan, the Experts walked me through the Excel version, which combines RegNav AI with their expertise.
Knowledge transfer:
Experts provided detailed explanations of CSV columns and terminology.
Insights into the hierarchical structure of the Compliance Plan content.
Design:
Imported CSV data into InDesign, its limitations acted as a valuable constraint.
Limitations forced critical evaluation of essential vs. non-essential information, enhancing our understanding of user needs.
Improving language, and naming conventions across the CSV and PDF versions.
CSV - Sample Compliance Plan.
InDesign build - Sample Compliance Plan.
Measure
We created user research scripts, combining customer interview and observational testing techniques, to validate user expectations, and identify missed opportunities. For these sessions, we engaged with users who had previously completed all the key RegNav stages ('Provide device details').
-
Gauging knowledge and confidence: User's existing knowledge and confidence related to the subject matter.
"What's your comfort level with the regulatory process? and how confident do you feel that you could get through it?"
Open-ended questions: Avoiding simple "yes" or "no" answers, encouraging the user to provide more detailed explanations.
“Can you recall a specific time when understanding or meeting regulatory requirements was particularly difficult or frustrating? What happened?"
Probing: Getting the user to elaborate on their initial responses.
"How do these challenges impact your project timelines, costs, or overall work? For example, where do you find yourself spending the most time or resources?"
Think aloud prompts: Encouraging the user to verbalise their thoughts as they interact with the material.
"What are you thinking as you go through all this?"
Specific inquiry: Asking for specific interpretations of elements within the process.
"What do you think those tags mean? The OLK and 510(k)?"
Expectation validation: Understanding if the output meets the user's expectations and identifying gaps.
"Looking back at the experience you've had with RegNav, is this what you expected as an output?"
Quote Examples
We do this (assessing if the product in development is compliant) in step 10. If we had this tool, we might be able to do it in step 1 or 2. So, it would've saved our efforts, time, energy and resources. Especially because after step 10 we might have had to go back to step 0.
“Needs more specificity about the testing that they can provide. This is pretty general info - if I'm looking for a testing lab, I know what kind of testing lab I am looking for, this does not tell me thatˮ
Compliance Plan Takeaways
Security layer: An early check within the development process. It's not replacing the entire process, but adding a crucial validation step to it.
Testing labs: More specific information on testing labs and what they can provide.
Add validation and human touch: Include authoritative statements, references to similar products, and reviewer credentials to build trust. Incorporate photos and human elements to make the document more relatable.
Enhance clarity and understanding: Provide educational content, clear explanations, and practical examples throughout the document.
Provide practical resources: Add legends and guides for interpreting detailed tables and Product Codes. Include example Compliance Plans and predicate device references to demonstrate practical application and accuracy.
Business Impact
The Static Compliance Plan, despite its limitations, served as an early customer-facing deliverable, securing our first paying customers.
More importantly, it was a crucial research tool that uncovered the true pain point in the compliance journey, and directly informed a significant market fit pivot.
Market Fit Pivot
Early enhancements, new Classification features, and a Static Compliance Plan directly impacted the RegNav launch. This enabled us to gain initial "Startup" traction, securing our first four paying customers at $10,000 per device.
Evolving Audience
Learning & validation: Observational testing, probing customer interviews, and metrics revealed a critical insight for the strategic pivot.
Strategic pivot: While Startups were excited about efficiency, Enterprise clients showed that their primary pain point was to mitigate the catastrophic risks of non-compliance, such as fines, product recalls, and going back to ground 0.
Why The Pivot?
Risk mitigation: Enterprises were most excited by the prospect of using AI as an additional layer of security, acting as a crucial checker in their compliance process.
Enhanced value: Dive deeper into the detail, extracting accurate Product-Level Requirements from Standards, providing a safeguard against costly compliance failures, and unlock lifecycle features like a Test Manager.
Market data & willingness to pay: Enterprises were willing to pay 2-3x more, with 30+ products in development. Data showed that 82% would trust a digital tool with human validation, with 60% likely to pay for an AI-driven solution.
Strategic acquisition: The pivot allowed us to leverage the Medical Experts niche Enterprise network, and the Element brand for customer acquisition.
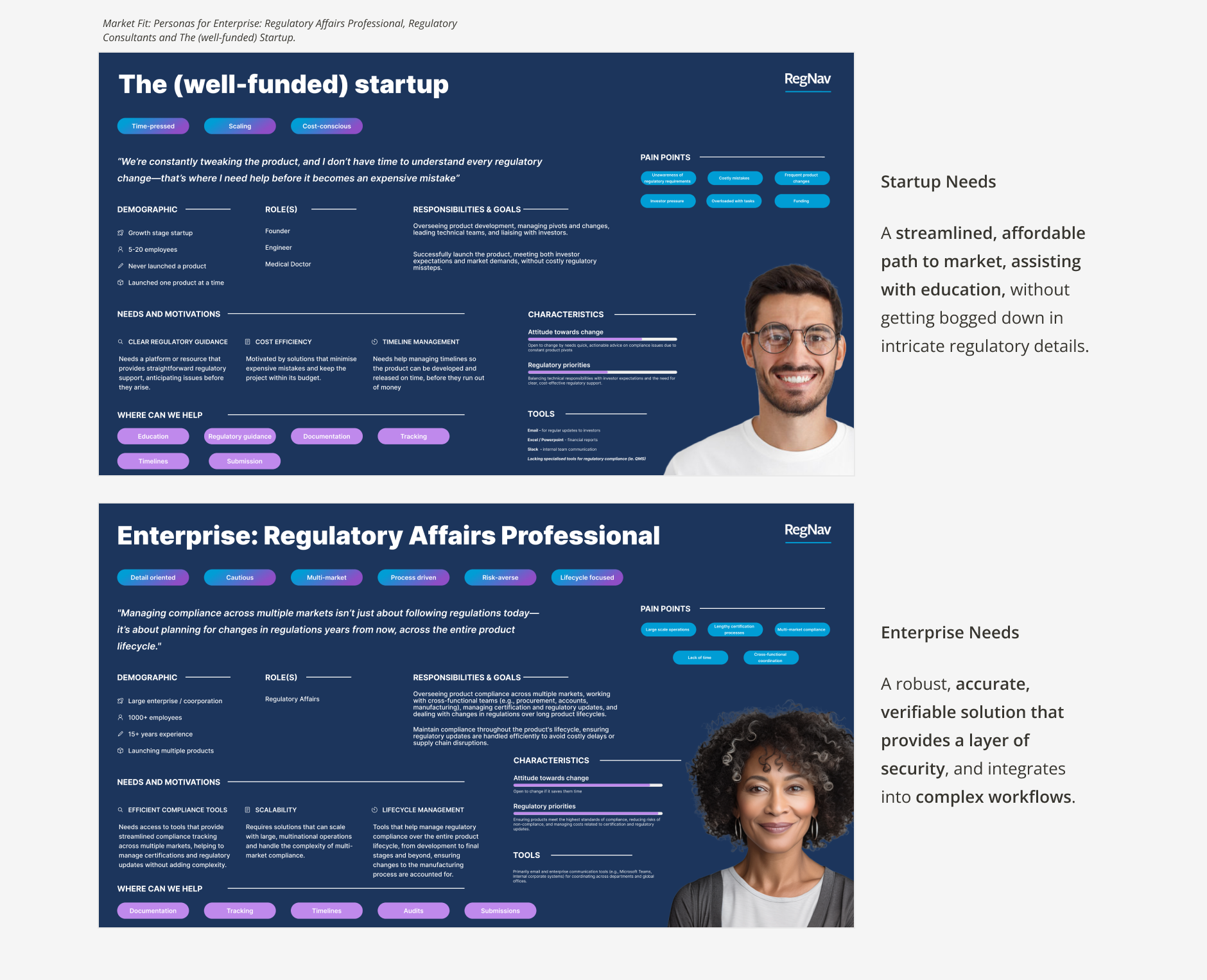
Personas
We continuously enriched our personas to reflect the specific needs and characteristics of our target audience, Enterprise: Regulatory Affairs Professionals.
Enterprise:
Detail oriented
Cautious
Multi-market
Process driven
Risk-averse
Lifecycle focused
Regulatory consultants:
Experienced
Multi-tasker
Analytical
Non-tech savvy
Resourceful
Relationship driven
The (well-funded) startup:
Time-pressed
Scaling
Cost-conscious
Customer Journey Map
To better serve the Enterprise persona, we mapped their end-to-end customer journey, from discovery to ongoing usage.
This process included their key actions, goals, and pain points.
Key updates to the map included, Multiple Markets, IT Security, Product-Level Requirements and adding a future Test Manager step, which reflected the Enterprise's needs for lifecycle management.
Product-Level Requirements
Problem Statement
Research revealed human error was a major risk in manual compliance, particularly when identifying applicable Requirements, as not all apply within a Standard. Crucially, accurate Requirement extraction is directly linked to unlocking key lifecycle features such as a Test Manager.
Key requirements:
Accurate extraction: Use AI to extract device specific Product-Level Requirements for a added layer of security.
Validate applicability: Intelligently filter out the ~30% of Requirements that are not applicable to a specific device.
Handle data volume: Our solution had to manage a substantial data load, as a single device's Compliance Plan could involve dozens of Standards, each containing hundreds of Requirements.
Plan distinction: Clearly distinguish between standard and enterprise-level plans by customising the UX/UI for each tier.
Anatomy of a Standard
Family: Describes the organisation that built, and maintains the Standard e.g. ISO, IEC.
Number: The unique identifier of that Standard within that family e.g. 60601.
Scope: Describes what the Standard talks about.
Clause: Sections of the Standard outlining Requirements.
Requirement: Specifies the criteria to comply with the Standard.
Condition: Specific conditions under which a Requirement applies. States yes/no whether the compliance with these Requirements is a must or optional.
Anatomy of a Standard and Clause.
AI-Driven Solution
The Challenge
The initial RegNav engine struggled to accurately extract Requirements from unstructured data (bulleted lists, tables), resulting in missed information from Standards.
To address the low accuracy, we developed a new multi-step process that integrated Large Language Models (LLMs) and a human-in-the-loop annotation tool.
AI extraction, missing Requirement.
AI extraction, Overall approach.
Refined Multi-Step Process
The refined pipeline successfully extracted the Requirements, continuously improved overall accuracy, and ensured a ‘human element’ remained a key user benefit, whilst the AI does the heavy lifting.
Engine: The core engine extracts Standards, Clauses, and Requirements.
Applicability (LLM): A Large Language Model was introduced to analyse the context of Standards, and classifies conditional Requirements.
Human-in-the-loop: Used the annotation tool Kili to manually validate and enrich the AI extracted conditions.
Ideate
Building on the existing Premium plan, I created user flows focused on key timeline events (e.g., requesting a Compliance Plan, receiving Standards and Requirements).
The work included defining progress statuses (Draft, Requested, Complete) and the types of communication methods (email, in-app alerts, progress visuals) to keep the user informed.
Mandatory Questionnaire Concept
The Data Science team indicated that customers must answer conditional questions to validate and uncover applicable Requirements, with the number of questions decreasing as the AI results cache improves.
I prototyped the new mandatory questionnaire flow, showing how it integrated with the existing navigation/stepper flow.
Pivot
Testing revealed that the mandatory questionnaire flow was overwhelming for users, with not enough context, hindering their progress.
We pivoted to embedding the conditional questions directly within an online Compliance Plan, allowing users to answer questions contextually at their convenience.
This pivot required negotiating a later delivery date, but the value of creating a better user experience and a scalable solution was worth the delay.
Progress Status & Timeline Events
This table highlights the key events between the different plans, and where the user benefits from the pivot to an Online Compliance Plan.
Progress status and timeline events chart.
Define & Build
Online Compliance Plan
A frictionless user journey, providing greater context to answer Requirement conditionality questions dynamically, all embedded within a scalable online Compliance Plan.
Dynamic & contextual questions: Presenting conditionality questions alongside their corresponding Requirements, allowing users to progress and complete the questions at their own pace in context, transforming a daunting up-front form into a elegant workflow.
Online Standards & Requirements: A scannable card view of each Standard and its associated Requirements, prioritising navigation for additional context, information hierarchy, and useful filters to manage the volume of the data
Status and notifications: Progress status indicators provide real-time updates, track completion and clear communication for customer awareness.
Streamlined features: Comprehensive filtering and consolidated questionnaire launcher, allowing users to quickly answer known questions and skip those they can't.
Measure
We received positive feedback when embedding the questions directly into the online Compliance Plan, validating our approach.
Product-Level Requirements Takeaways
Supporting visuals: Provide images and diagrams for additional context.
Custom filtering: Implement Excel-like views.
Volume of questions: Continue to enrich the AI's results cache, reducing the number of conditionality question.
Dynamic conditionality questions user testing session.
Conclusion
Results & Impact
The Product Code Finder, and Online Compliance Plan incorporating Product-Level Requirements, were successfully delivered.
RegNav slashed the time create a Compliance Plan by 40%, from 150+ hrs to 90 hrs.
Reduced the risk of human error, providing an AI-driven compliance checker, with Requirement level accuracy that unlocked future lifecycle features such as the Test Manager.
We secured our first four Startup customers, and following a strategic market fit pivot acquired our first Enterprise customer.
Due to a company-wide decision to optimise spending, I was unable to continue my work on both the online Compliance Plan testing, and the Test Manager build.
My contributions in UX/UI helped bridge the gap between user behaviour, and business strategy. The robust foundations we established will serve as invaluable assets for RegNav's future growth.
Key Learnings
Robust foundations: Shared documentation and system design, to surface patterns and build efficiently.
User research to validate pivots: Framing the Static Compliance Plan as a research tool that uncovered critical insights, and justified our strategic shift.
Strategic agility: The willingness to change course, pivoting from saving costs for startups, to mitigating catastrophic risk for enterprises unlocking the product’s true value.